Neat Info About Can Electrons Flow Freely

Electricity Unveiled
1. The Free Electron Concept
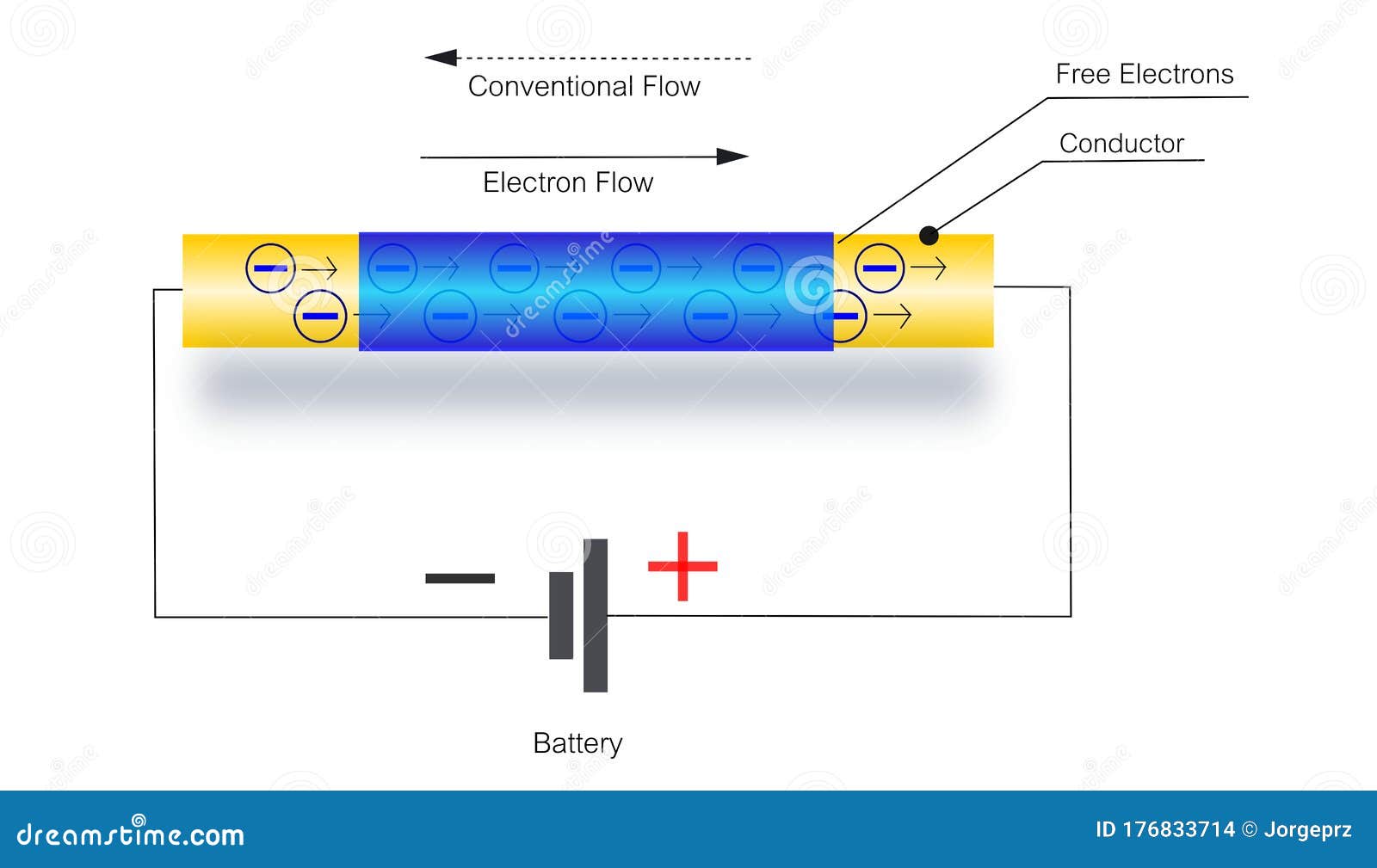
Ever wondered how that light bulb magically illuminates a room? Or how your phone manages to binge-watch cat videos for hours? It all boils down to electrons — those tiny, negatively charged particles zipping around atoms. But here's the kicker: not all electrons are created equal when it comes to freedom of movement. Some are tightly bound to their atoms, like velcro stuck to a wall, while others are more like that one friend who's always ready for an adventure. These adventurous ones are what we call "free electrons," and their ability to wander determines how well a material conducts electricity.
Think of it like a crowded dance floor. If everyone's glued to their spot, there's no movement, no energy transfer. But if some people are moving around, bumping into others, and generally causing a ruckus, energy spreads across the room. Similarly, free electrons bumping into atoms and other electrons are what create electrical current. The more free electrons, the easier it is for current to flow. And that, my friends, is the basic principle behind electrical conductivity.
Now, you might be thinking, "So, what makes an electron 'free' anyway?" Well, it's all about the atomic structure of the material. In some materials, like metals (think copper wiring), the outermost electrons aren't strongly attached to the nucleus. They can easily detach and roam throughout the material. In other materials, like rubber or glass, the electrons are tightly held, making it difficult for them to move freely. That's why rubber is an excellent insulator — it resists the flow of electricity.
It's fascinating to consider that the very fabric of our modern technological world relies on the seemingly simple ability of these tiny particles to, well, electron-ically "hang loose". Without those free electrons, we'd be stuck in the dark, with no cat videos to distract us from the existential dread of our unconnected lives.

Conductors, Insulators, and Semiconductors
2. The Spectrum of Electrical Flow
Now that we've established the importance of free electrons, let's delve into the different types of materials based on their ability to conduct electricity. We can broadly categorize them into three main groups: conductors, insulators, and semiconductors. Each has a unique "free electron" personality.
Conductors are the rockstars of electrical conductivity. Materials like copper, silver, and gold are teeming with free electrons, making them excellent at conducting electricity. Imagine a highway packed with speedy cars — that's what it's like inside a conductor when electrons are moving through it. These materials are used in everything from power lines to the intricate wiring inside your computer.
Insulators, on the other hand, are the party poopers of the electron world. They have very few free electrons, making it difficult for electricity to flow through them. Think of them as a tightly guarded fortress, preventing any electrical current from passing. Materials like rubber, glass, and plastic are excellent insulators and are used to protect us from electric shocks.
Then there are semiconductors, the intriguing middle ground. These materials, like silicon and germanium, have conductivity between that of conductors and insulators. Their conductivity can be controlled by adding impurities (a process called doping) or by applying an electric field. This makes them incredibly versatile and essential for building transistors, which are the building blocks of modern electronics. So, semiconductors aren't just sitting around; they are more like a bouncer determining who gets in and who does not.

10. Diagram Of An Electron Leaving Atom And The Subsequent Moving
Factors Influencing Electron Flow
3. Temperature, Impurities, and Other Electron-Flow Interrupters
Even in the best conductors, the flow of electrons isn't always a smooth, uninterrupted ride. Several factors can influence how easily electrons can move through a material. It's like driving on a road with potholes, traffic jams, and unexpected detours.
Temperature plays a significant role. As temperature increases, the atoms in a material vibrate more vigorously, which can impede the movement of electrons. Think of it like trying to run through a crowded room where everyone is bumping into you. This is why the resistance of most conductors increases with temperature. Conversely, some materials, like semiconductors, exhibit increased conductivity at higher temperatures, adding to their unique properties.
Impurities can also affect electron flow. Introducing foreign atoms into a material can disrupt the regular arrangement of atoms, creating obstacles for electrons to navigate. These impurities can either scatter electrons or, in the case of semiconductors, provide additional electrons (or "holes," which act as positive charge carriers) that enhance conductivity.
The material's structure itself matters. In perfectly crystalline materials, with atoms arranged in a highly ordered pattern, electrons can move more freely. However, defects in the crystal structure, such as grain boundaries or dislocations, can scatter electrons and increase resistance. Imagine a perfectly smooth highway versus one with cracks and bumps — the smoother the road, the faster the ride.
Understanding these factors is crucial for designing and optimizing electronic devices. Engineers carefully select materials and control their properties to ensure that electrons flow efficiently and reliably, enabling our gadgets to function as intended.
Superconductivity
4. The Ultimate Electron Flow
Now, let's talk about something truly extraordinary: superconductivity. Under certain conditions, some materials exhibit a phenomenon where their electrical resistance drops to zero. Yes, you read that right — zero resistance! This means that electrons can flow through these materials without losing any energy. It's like electrons dancing in perfect harmony, gliding effortlessly without any friction.
Superconductivity typically occurs at extremely low temperatures, often near absolute zero (-273.15C or -459.67F). At these temperatures, electrons pair up to form what are called "Cooper pairs." These pairs behave like a single entity and can move through the material without being scattered by atoms. It's as if the electrons have found a secret cheat code to avoid all obstacles.
The implications of superconductivity are enormous. Imagine lossless power transmission, where electricity can be transported across vast distances without any energy loss. Or ultra-fast computing, where electronic devices can operate at speeds previously unimaginable. Superconducting magnets are already used in MRI machines, and research is ongoing to develop new superconducting materials that can operate at higher temperatures.
While practical applications of superconductivity are still limited by the need for extremely low temperatures, the potential benefits are so significant that scientists continue to push the boundaries of materials science in the quest for room-temperature superconductors. It's a bit like the pursuit of the Holy Grail, but with electrons.
Elektronit Ja" Reiät " Kiinteän Olomuodon Laiteteoria Elektroniikan
Beyond the Wire
5. Electron Flow in Everyday Life
So, "Can electrons flow freely?" The answer, as we've seen, is a resounding "it depends!" It depends on the material, the temperature, the presence of impurities, and even the way the material is structured. But what's truly remarkable is how this fundamental principle of electron flow underpins so much of our modern world.
From the simple act of flipping a light switch to the complex operations of a supercomputer, everything relies on the controlled movement of electrons. The next time you use your smartphone, consider the intricate dance of electrons within the microchips, making it all possible. Every time you watch TV, think about how the electricity flowing through the cables is powered by electrons moving freely within the wires.
Even phenomena like lightning are essentially giant displays of electron flow in the atmosphere. It is a natural reminder of the raw power and energy unleashed when electrons overcome resistance and discharge in a dramatic display.
Ultimately, understanding the principles of electron flow is key to unlocking new technological advancements. By manipulating materials at the atomic level, scientists and engineers can create devices with unprecedented capabilities, leading to innovations we can only dream of today. So, next time you're pondering the mysteries of the universe, remember those tiny electrons, busily zipping around and making it all happen.